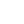
Florfenicol 10% Injection
|
Function |
Antibacterial Drugs |
|
|
Product Name |
Florfenicol 10% Injection |
|
|
Composition |
Each ml contains florfenicol 100mg. |
|
|
Appearance |
colorless or almost colorless, liquid, slightly viscous |
|
|
Indication |
Antipyretic,antalgic agent. Treatment for muscular pain, hernial pain, rheumatism and fever diseases. It has strong fever relieving effects, anti-inflammatory effect and strong analgesic effect. |
|
|
Dosage and Administration |
Pigs, chicken: 0.2 ml for 1kg body weight. Dosage interval: 48 hours for once, continue for 2 times. |
|
|
Side Effects |
Forbidden in the milk producing period and egg laying hens. |
|
|
Precaution |
①For livestock of renal insufficiency reduce dose or extend dosing intervals . ②Forbidden in vaccination period or serious immune function disable . |
|
|
Withdrawal Period |
Pig: 14 days; chicken: 28 days. |
|
|
Productivity |
40000 bottles per day |
|
|
Brand |
TIANYUAN |
|
|
Transportation |
Ocean,Land,Air |
|
|
Place of Origin |
Hebei, China |
|
|
Certificate |
GMP |
|
|
HS Code |
300490 |
|
|
MADE IN CHINA |
|
|
Thank you for your visit! We provide various specifications of veterinary drug products,
accept custom packaging.All our production lines have passed GMP verification and are
safe and reliable. Welcome to contact us for win-win cooperation
Recommended Products
天元药业
Tianyuan Pharmaceutical
Add: No. 34, Changsheng Street, Luquan District, Shijiazhuang City, Hebei Province Tel:0086-311-83980006/83980378 Fax:0086-311-83980378 E-mail:ty@tianyuanyaoye.com.cn